The Sauvageau Lab aims to identify novel strategies to expand HSCs for the treatment of hematological malignancies.
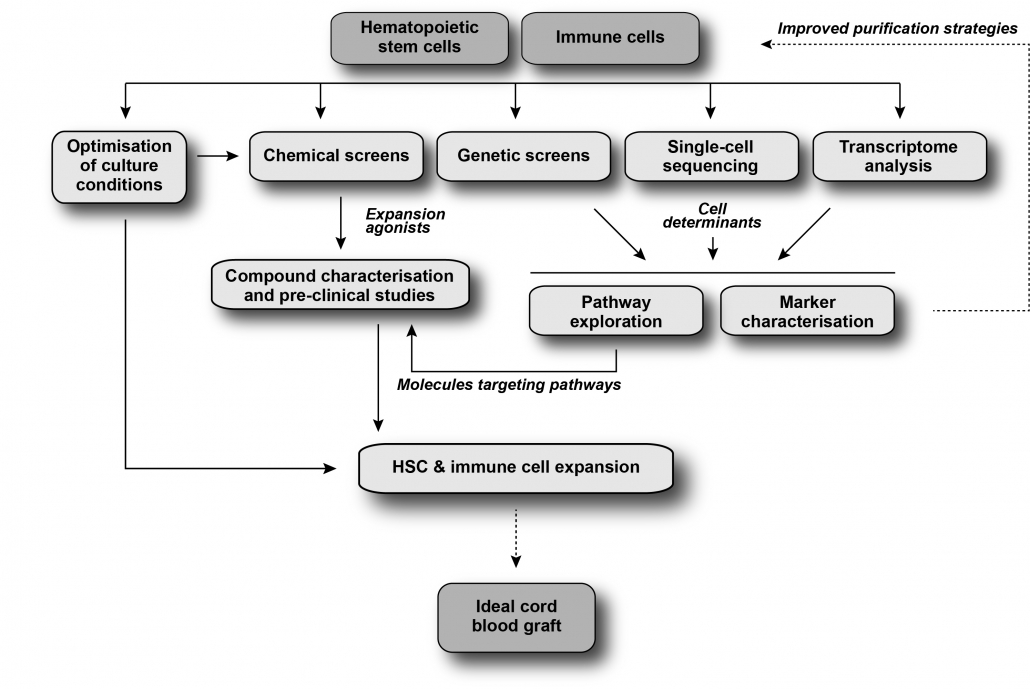
Hematopoietic stem cells (HSCs) have the ability to self-renew, allowing sustained lifelong production of all mature blood cells in vivo, and to produce progenitors that ensure the daily supply of all essential blood components. Given their unique properties, HSCs are widely used in transplantation to cure many blood diseases and malignancies, but unfortunately 40% of patients lack the required human leukocyte antigen (HLA)-matched donor. Because of their immaturity, cord blood (CB) grafts allow for multiple HLA mismatches and this permissiveness increases access to HSC transplants to more than 95% of patients. Moreover, when compared to other grafts, CB transplants are associated with less chronic graft-vs-host-disease (GVHD) and possibly with fewer incidences of relapse of the underlying malignancy when compared to other grafts. Currently, the main issue associated with CB transplantation is the low cell dose in cord blood units, which results in delayed or absence of engraftment, thereby increasing early mortality. As a result, there is great interest in molecules that expand HSCs.
Our lab, in collaboration with Anne Marinier’s group at IRIC, has identified the small molecule UM171, a compound that enhances the expansion of human CB HSCs (Fares et al., Science, 2014 and Pabst et al., Nature Methods, 2014). The benefits of UM171-mediated expansion of CB HSCs for HSC transplantation are currently being investigated as part of clinical trials for many hematological diseases. This research program aims to identify additional innovative strategies to further improve HSC expansion, to optimize HSC graft composition and translate these discoveries into clinical applications for AML treatment.
Chemogenomic dissection of HSC self-renewal networks
The Sauvageau Lab has a longstanding interest in the identification and characterization of functional determinants of HSC self-renewal. Previous studies enabled our group to unveil key genetic regulators of this process in the mouse system (Deneault et al., Cell, 2009; Hope et al., Cell stem cell, 2010; Cellot et al., Blood 2013). We are currently pursuing this initiative by extending our focus to human HSCs using optimized culture conditions involving the small molecule UM171. This effort led to the identification of several human HSC markers (EPCR : Fares et al., Blood, 2017; ITGA3 : Tomellini et al., Cell reports, 2019; HLF : Lehnertz et al., 2021). Our overall strategy involves integrated cellular, genetic and pharmacological approaches with which we hope to shed light on molecular regulators or pathways that impact human HSC self-renewal and differentiation in culture. Eventually we hope to genetically or pharmacologically target these regulators to further improve HSC self-renewal ex vivo for therapeutic purposes.
Defining the ideal expanded cord blood graft
A major challenge in HSC transplantation is providing grafts that will secure a rapid and sustained engraftment with optimal anti-leukemic activity and minimal side effects. Delayed recovery of the immune system is a major cause of transplant-related mortality and graft-versus-host disease (GVHD). Moreover, a growing body of evidence shows that the innate immune system makes a crucial contribution to the graft tolerance and anti-tumor activity. Consequently, regeneration of an appropriate and potent immune system remains an important clinical need in the context of HSC transplantation. This project focuses on dissecting the biology and identifying the main determinants and effectors of GVHD and graft-versus-leukemia (GVL). Using both genetic and chemical approaches, we wish to develop strategies to expand appropriate immunomodulatory cells to produce HSC grafts with optimal cell immunity, and consequently with reduced associated risk of GVHD and high anti-viral and GVL properties.
HSC expansion to the clinic
The ability of the small molecule UM171 to expand human cord blood derived HSCs is being translated to the clinic with assistance from expert clinician scientists. A phase I/II clinical trial was launched in 2016 to assess the benefits of UM171-expanded cord blood cell transplantation for the treatment of hematological diseases including acute myeloid leukemia. Results from this study were published in Lancet Haematology in 2019 and show that the transplantation of UM171-expanded cord blood cells is safe, opening the door to the transplantation of cells from smaller, but more compatible, CB units, hence allowing to exploit the advantages associated with cord blood grafts compared to other stem cell sources.